Uruguay – CRO Services – Clinical Trial Application Support
Simplified Roadmap
QMS-SR-RA-MSP-clinical-trial-applicati
Version: 08/20/2025
Prepared by: Dr. Vlad Reznikov
Date: August 19, 2025
Executive Summary
This roadmap describes Clinical Trial Application Support in Uruguay for the CRO Services category. Consider market size, population, GDP and PPP when prioritizing launch order—see GDP Matrix (ROCKSTARS, HONEYBEES, MAVERICKS, UNDERDOGS): https://patternofusa.com/gdpmatrix/. Peer markets with similar frameworks may allow reliance or abridgement where eligible.
Key Regulations & Requirements
National Regulatory Authority (NRA): MSP
Documents / Standards / Certifications:
- CTA/IND pack
- Protocol & IB
- Ethics approvals
- Safety plan
- Data mgmt & stats plan
Notes: Submission format, portal, legalization, and language for Uruguay require verification .
Estimated Timeline
Zero‑based Gantt timeline (weeks). Bars are illustrative; adjust per project scope.
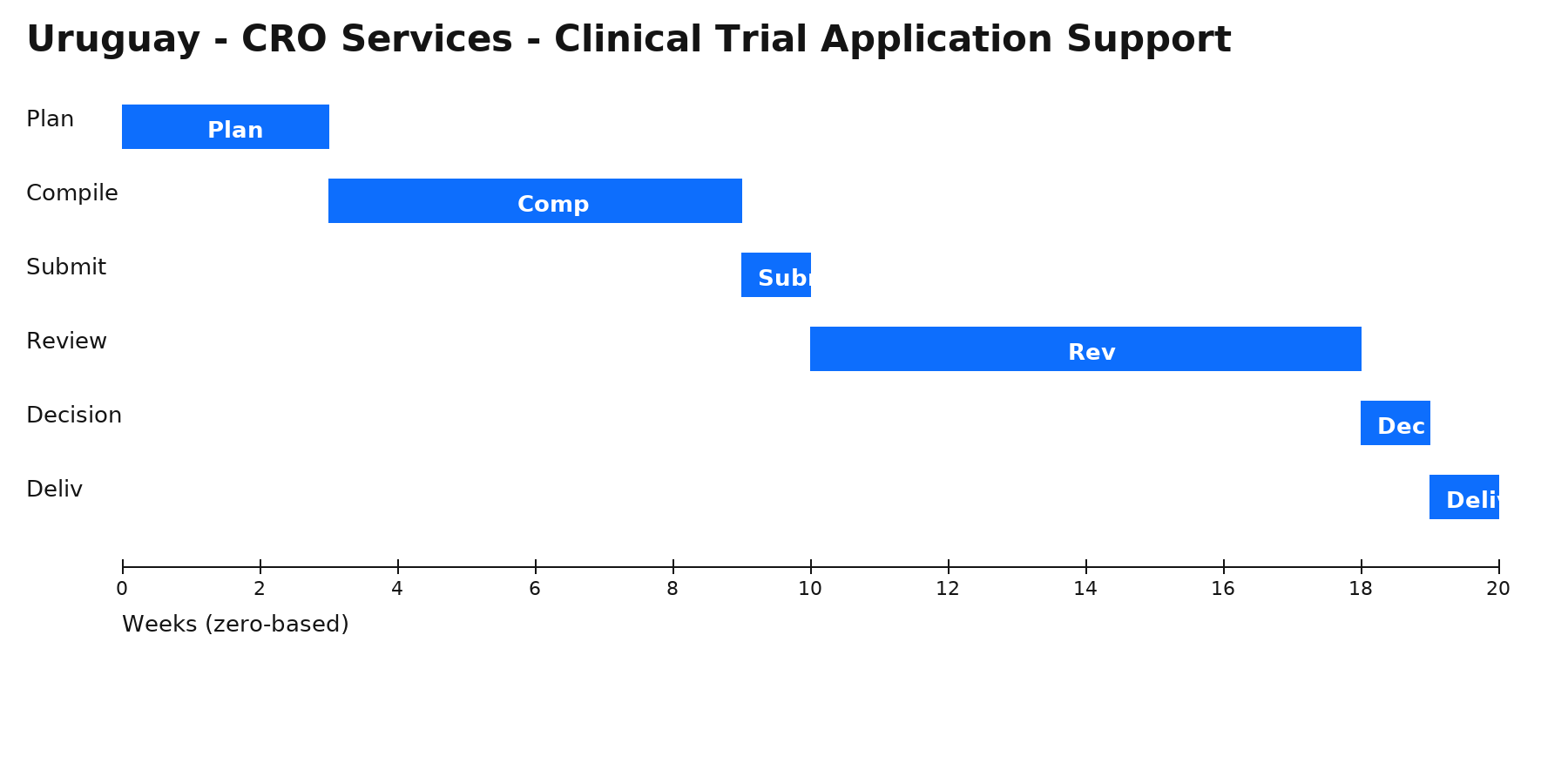
Phases & Tasks
Plan (3 wks) — Define pathway, reliance, local roles (MAH/agent); build high-level plan & risk register.
Compile (6 wks) — Assemble dossier/quality evidence; confirm translations, legalization/apostille, and samples/testing.
Submit (1 wk) — File via portal/gateway; complete fee payment and resolve validation issues.
Review (8 wks) — Manage queries/clock-stops; submit clarifications, addenda, or product samples as requested.
Decision (1 wk) — Receive authorization or additional requests; align launch, PV, and supply readiness.
Deliv (1 wk) — Issue internal release pack (certificates, approved labels, SOPs) and plan for renewals/variations.
Deliverables
- Authorization/Certificate or Registration Record
- Approved labeling/artwork (as applicable)
- Final dossier, correspondence log, and post‑approval plan
