Kazakhstan – Pharmaceuticals or Small-Molecule Drugs – Marketing Authorization (New Drug)
Simplified Roadmap
QMS-SR-RA-NCEMMD-marketing-authorization-new-drug
Version: 08/20/2025
Prepared by: Dr. Vlad Reznikov
Date: August 19, 2025
Executive Summary
Kazakhstan participates in the EAEU common market for medicines. New registrations follow the EAEU MRP/DCP framework, with national expertise performed by the state expert organization. Legacy national registrations are being brought into compliance within the EAEU transition window. Sponsors should plan RMS/recognition-state strategy, PV/QMS readiness, and local administrative steps coordinated with the expert body.
Key Regulations & Requirements
Authorities in KZ: Ministry of Health; state expert organization National Center for Expertise of Medicines and Medical Devices (often abbreviated NCEMMD/NDDA) — conducts scientific and technical expertise for state registration and variations.
Legal basis (applied in KZ): EEC Council Decision No. 78 (EAEU registration & expertise rules); EEC Council Decision No. 77 (EAEU GMP) and Pharmaceutical Inspection Rules (Decision No. 83); EAEU GVP (Decision No. 87); EAEU Pharmacopoeia. National procedures cover price registration and other state controls where applicable.
Dossier / standards:
- eCTD/CTD M1–M5 per EAEU; SmPC/leaflet per EAEU decision; translations as required;
- GMP evidence (EAEU GMP); samples/reference standards/tests if requested by the expert organization;
- PV system description and local contact arrangements;
- Administrative forms/contracts with the expert organization and fee payment slips;
- Separate state price registration where applicable to reimbursement/procurement.
Estimated Timeline
Zero-based Gantt timeline (weeks). Bars are illustrative; adjust per project scope.
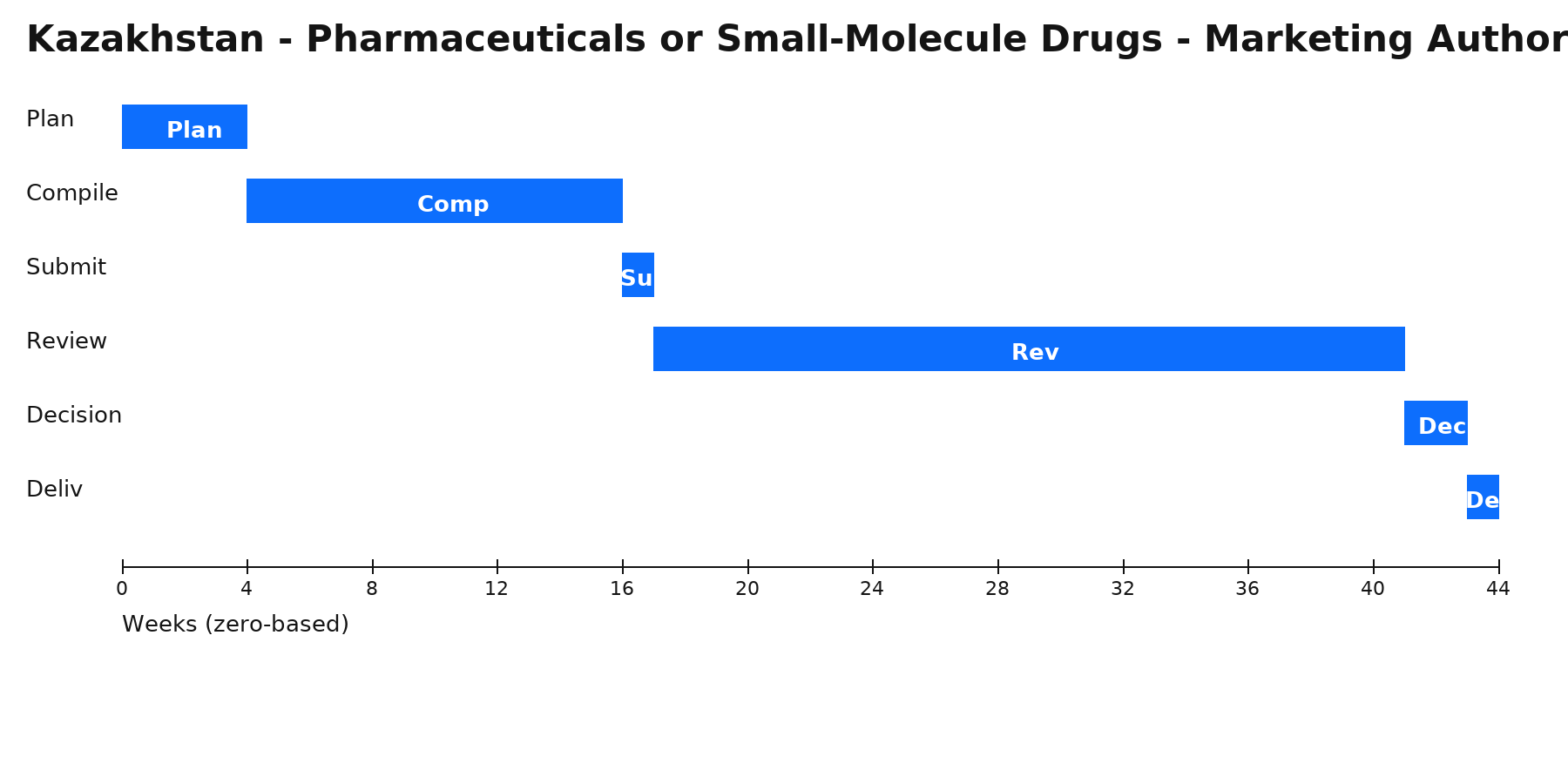
Phases & Tasks
Plan (4 wks) — Confirm EAEU route (MRP or DCP), RMS/recognition-state plan, and KZ expertise logistics (contracting with the expert organization); align PV and GMP inspection status.
Compile (12 wks) — Build eCTD M1–M5; ensure CMC/quality meets EAEU GMP and pharmacopoeial specs; prepare SmPC/leaflet; prepare responses to likely quality/clinical LoQs.
Submit (1 wk) — File in RMS and initiate recognition in KZ (MRP) or include KZ in the DCP set; settle fees; pass technical validation.
Review (24 wks) — Kazakhstan’s expert organization performs scientific/technical expertise alongside RMS/recognition timelines; manage clock-stops and provide data/samples as requested.
Decision (2 wks) — Issuance of MA per EAEU model in KZ; finalize post-decision administrative steps and listings.
Deliv (1 wk) — Internal release: MA certificate, approved SmPC/leaflet, PV obligations, renewal/variation calendar; complete price registration where applicable for public procurement.
Deliverables
- Kazakhstan MA aligned to EAEU format (RMS/recognition outcome);
- Approved SmPC/leaflet/artwork; final eCTD; correspondence record;
- PV/RMP commitments; pricing registration (if applicable) and post-approval plan.
