Eurasian Economic Union – EAEU – Pharmaceuticals or Small-Molecule Drugs – Marketing Authorization (New Drug)
Simplified Roadmap
QMS-SR-RA-EEC-marketing-authorization-new-drug
Version: 08/20/2025
Prepared by: Dr. Vlad Reznikov
Date: August 19, 2025
Executive Summary
This roadmap summarizes how to obtain an EAEU-wide marketing authorization (MA) for human medicinal products via the Mutual Recognition Procedure (MRP) or the Decentralized Procedure (DCP) under the Union’s common rules. Sponsors select a Reference Member State (RMS) for first scientific assessment (MRP), then expand to chosen Recognition States; or file simultaneously in all selected states (DCP). New registrations proceed under EAEU rules, while legacy national MAs must be brought into full compliance by the end of the transition period.
Key Regulations & Requirements
Union legal basis: EEC Council Decision No. 78 (Rules of Registration & Expertise of Medicines) — establishes MRP/DCP, dossier and assessment framework; EEC Council Decision No. 77 (EAEU GMP); EAEU Pharmaceutical Inspection Rules (EEC Council Decision No. 83); EAEU Good Pharmacovigilance Practice (Decision No. 87); EAEU Pharmacopoeia; and updates to EAEU Good Clinical Practice. Electronic format requirements for applications are also defined at Union level.
Dossier / standards:
- eCTD/CTD Modules 1–5 in the RMS (Union format); Module 1 per EAEU specifics (SmPC/leaflet requirements per EAEU decision);
- Quality (CMC) compliant with EAEU GMP (Decision 77) and EAEU Pharmacopoeia where applicable;
- Nonclinical and clinical summaries (BE/efficacy/safety as appropriate);
- PV system & commitments per EAEU GVP; risk management (RMP) where required;
- Administrative forms, fees, MAH/authorized representative, samples and reference standards if requested;
- Electronic submission per EAEU requirements.
Transition / reliance: New registrations proceed under EAEU rules; previously national MAs must be converted/updated to EAEU format within the transition timeframe defined by EAEU decisions and notices.
Estimated Timeline
Zero-based Gantt timeline (weeks). Bars are illustrative; adjust per project scope.
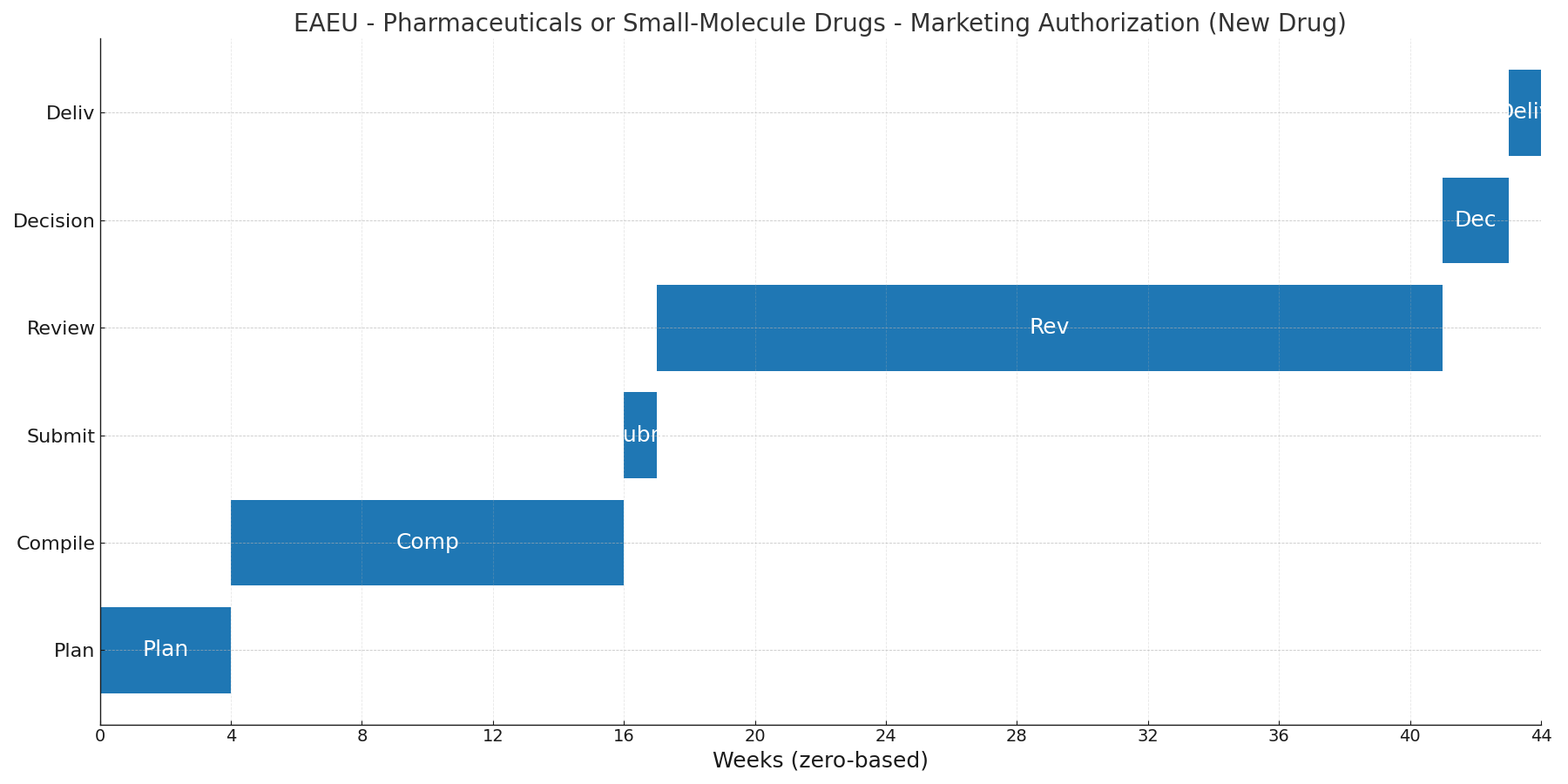
Phases & Tasks
Plan (4 wks) — Determine MRP vs DCP; pick RMS; confirm recognition states; align dossier granularity; confirm GMP inspection needs and PV set-up.
Compile (12 wks) — Prepare eCTD M1–M5 per Decision 78; finalize SmPC/leaflet per EAEU requirements; verify pharmacopoeial specs; ensure GMP evidence and, if needed, schedule/close GMP inspections.
Submit (1 wk) — File to RMS (and recognition states for DCP); pay fees; resolve technical validation and admin queries.
Review (24 wks) — Scientific/quality/clinical assessment in RMS; respond to LoQs and clock-stops; proceed to recognition states (MRP) and close any national conditions.
Decision (2 wks) — Receive EAEU-format MA in RMS and recognition states as applicable; complete national formalities (certificate issuance where applicable).
Deliv (1 wk) — Internal release pack: MA certificate(s), approved SmPC/leaflet, PV obligations, serialization/track & trace if applicable, renewal calendar.
Deliverables
- EAEU-compliant MA (RMS + recognition states, or DCP set);
- Approved SmPC/leaflet/artwork; final eCTD; correspondence log;
- PV/RMP commitments and post-approval plan.
